China Resources Zizhu tiene una línea de producción de medicamentos e instrumentos para la salud reproductiva, así como para medicamentos orales, medicamentos oftalmológicos, medicamentos para la diabetes, medicamentos de anestesia local, api química, etc. En cuanto al mercado nacional, la píldora anticonceptiva de emergencia (Comprimidos de Levonorgestrel) y el medicamento contra el embarazo precoz (Comprimidos de Mifepristona) ocupan la mayor proporción en el mercado chino, y sus comprimidos de levonorgestrelson es la marca líder de venta de medicamentos en la plataforma O2O del comercio electrónico en los últimos años. En el mercado internacional, China Resources Zizhu ha conseguido la certificación PQ de la Organización Mundial de la Salud y la certificación GMP de WHO Y PICs. Es un proveedor de medicamentos de salud reproductiva para las Naciones Unidas y las principales organizaciones internacionales, y su Levonorgestrel api ha sido pionero en cuento a la cuota en el mercado de exportación, y las exportaciones de comprimidos de Mifepristona y Misoprostol también se cuentan entre los primeros en China. Cumpliendo con el propósito corporativo de "China saludable, Vida mejor", la empresa aumenta constantemente la inversión en la investigción y el desarrollo, enfocándose en la innovación científica y tecnológica, arando profundamente el campo de la salud reproductiva, especialidades especiales y negocios internacionales para buscar la excelencia y perseguir el espíritu de ingeniosidad, ¡y se esfuerza por abrir un nuevo capítulo en el desarrollo a pasos agigantados!

2019.11

2019.11
WHO GMP
Co-package of Mifepristone Tablets and Misoprostol Tablets
2018.12
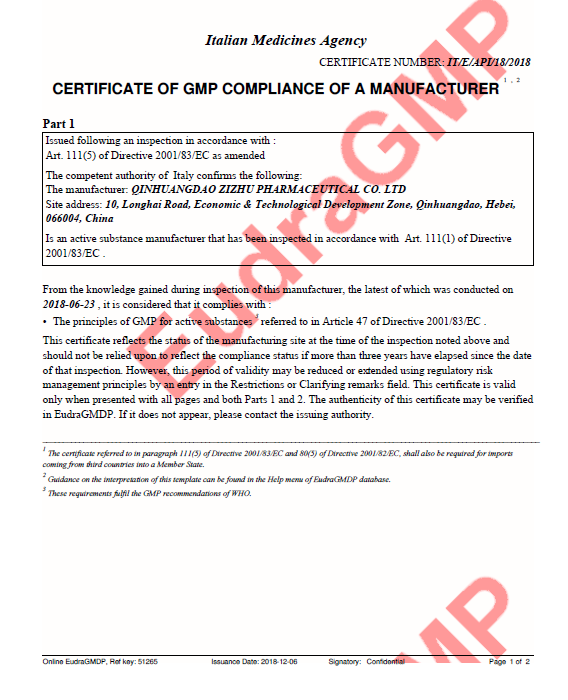
2018.12
EU GMP
Levonorgestrel API
2018.10

2018.10
EDQM GMP
Levonorgestrel API
2016.10

2016.10
PIC/S GMP
Misoprostol Tablets and Mifepristone (200mg)Tablets
2016.09

2016.09
Mexico COFEPRIS
Misoprostol Tablets
2016.01

2016.01
WHO GMP
Misoprostol Tablets and Mifepristone (200mg)Tablets
2013.09

2013.09
Mexico COFEPRIS
Levonorgestrel,Mifepristone,Ethinyl Estradiol API
2012.05

2012.05
WHO GMP
Levonorgestrel,Mifepristone,Ethinyl Estradiol API








