China Resources Zizhu Pharmaceutical имеет линейки продукции, включающие препараты и устройства для репродуктивного здоровья, пероральные препараты, офтальмологические препараты, диабетические препараты, препараты для местной анестезии и химическое сырье. На внутреннем рынке доля экстренных контрацептивов (таблетки левоноргестрела) и препаратов против беременности (таблетки мифепристона) является одной из самых высоких, а таблетки левоноргестрела являются ведущим брендом в продажах лекарств на платформах электронной коммерции O2O в последние годы. Как поставщик левоноргестрела, компания занимает ведущую долю экспортного рынка в Китае, а ее экспорт таблеток мифепристона и мизопростола занимает одно из первых мест в Китае. С корпоративным девизом "Здоровый Китай, хорошая жизнь",China Resources Zizhu Pharmaceutical продолжает инвестировать в исследования и разработки, фокусироваться на научно-технических инновациях, культивировать область репродуктивного здоровья, специальностей и международного бизнеса с профессионализмом и мастерством, стремясь к новой главе скачкообразного развития!

2019.11

2019.11
WHO GMP
Co-package of Mifepristone Tablets and Misoprostol Tablets
2018.12
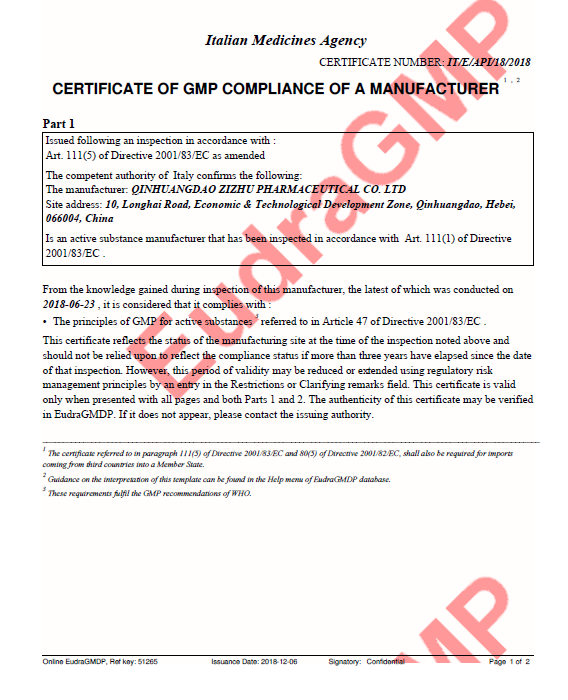
2018.12
EU GMP
Levonorgestrel API
2018.10

2018.10
EDQM GMP
Levonorgestrel API
2016.10

2016.10
PIC/S GMP
Misoprostol Tablets and Mifepristone (200mg)Tablets
2016.09

2016.09
Mexico COFEPRIS
Misoprostol Tablets
2016.01

2016.01
WHO GMP
Misoprostol Tablets and Mifepristone (200mg)Tablets
2013.09

2013.09
Mexico COFEPRIS
Levonorgestrel,Mifepristone,Ethinyl Estradiol API
2012.05

2012.05
WHO GMP
Levonorgestrel,Mifepristone,Ethinyl Estradiol API








